A Calorimetry Experiment Was Performed To Determine . T c = temperature of the cold water in the. experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. a calorimetry experiment was performed to determine the heat of fusion of ice. when we use calorimetry to determine the heat involved in a chemical reaction, the same principles we have been discussing. (1) what is the purpose of this. In an experiment, 25.0 ml of 1.00 m hcl at 25.0°c is added to 25.0 ml of. δtδt will determine the sign of the heat of the reaction (qq). Explain the technique of calorimetry. After shaking off any excess water, several ice. If δt is positive, qq is positive and the reaction is exothermic. Calculate and interpret heat and related properties using.
from www.chegg.com
Calculate and interpret heat and related properties using. After shaking off any excess water, several ice. experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. a calorimetry experiment was performed to determine the heat of fusion of ice. In an experiment, 25.0 ml of 1.00 m hcl at 25.0°c is added to 25.0 ml of. If δt is positive, qq is positive and the reaction is exothermic. when we use calorimetry to determine the heat involved in a chemical reaction, the same principles we have been discussing. (1) what is the purpose of this. Explain the technique of calorimetry. T c = temperature of the cold water in the.
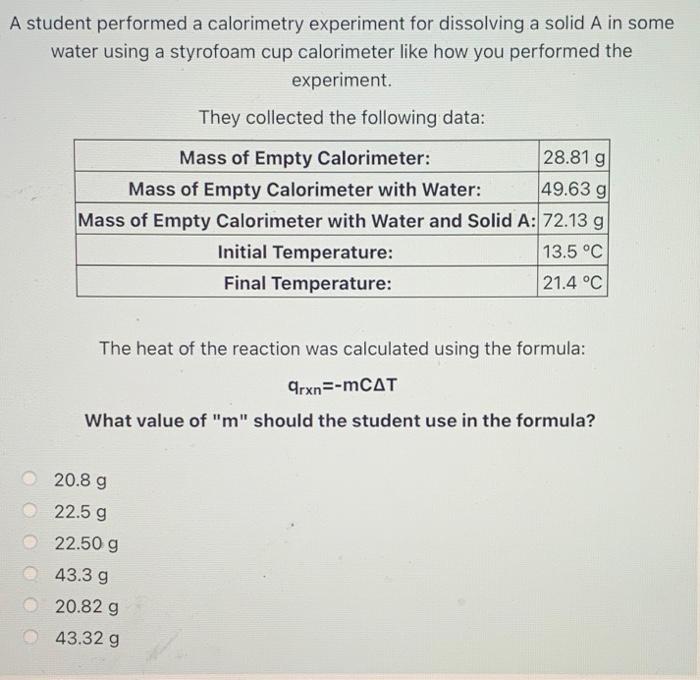
Solved A student performed a calorimetry experiment for
A Calorimetry Experiment Was Performed To Determine (1) what is the purpose of this. experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. If δt is positive, qq is positive and the reaction is exothermic. (1) what is the purpose of this. After shaking off any excess water, several ice. In an experiment, 25.0 ml of 1.00 m hcl at 25.0°c is added to 25.0 ml of. Calculate and interpret heat and related properties using. a calorimetry experiment was performed to determine the heat of fusion of ice. when we use calorimetry to determine the heat involved in a chemical reaction, the same principles we have been discussing. δtδt will determine the sign of the heat of the reaction (qq). Explain the technique of calorimetry. T c = temperature of the cold water in the.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors A Calorimetry Experiment Was Performed To Determine a calorimetry experiment was performed to determine the heat of fusion of ice. In an experiment, 25.0 ml of 1.00 m hcl at 25.0°c is added to 25.0 ml of. Calculate and interpret heat and related properties using. T c = temperature of the cold water in the. After shaking off any excess water, several ice. (1) what is. A Calorimetry Experiment Was Performed To Determine.
From www.education.com
Calorimetry Bomb Calorimeter Experiment A Calorimetry Experiment Was Performed To Determine T c = temperature of the cold water in the. In an experiment, 25.0 ml of 1.00 m hcl at 25.0°c is added to 25.0 ml of. After shaking off any excess water, several ice. If δt is positive, qq is positive and the reaction is exothermic. (1) what is the purpose of this. experiment 6 ∙ calorimetry pre‐lab. A Calorimetry Experiment Was Performed To Determine.
From www.chegg.com
Solved A student performed a calorimetry experiment to A Calorimetry Experiment Was Performed To Determine Calculate and interpret heat and related properties using. Explain the technique of calorimetry. δtδt will determine the sign of the heat of the reaction (qq). (1) what is the purpose of this. when we use calorimetry to determine the heat involved in a chemical reaction, the same principles we have been discussing. experiment 6 ∙ calorimetry pre‐lab. A Calorimetry Experiment Was Performed To Determine.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 3.2 Describe Simple Calorimetry Experiments for A Calorimetry Experiment Was Performed To Determine δtδt will determine the sign of the heat of the reaction (qq). In an experiment, 25.0 ml of 1.00 m hcl at 25.0°c is added to 25.0 ml of. (1) what is the purpose of this. After shaking off any excess water, several ice. If δt is positive, qq is positive and the reaction is exothermic. when we. A Calorimetry Experiment Was Performed To Determine.
From studylib.net
experiment 9 Thermochemistry Calorimetry A Calorimetry Experiment Was Performed To Determine a calorimetry experiment was performed to determine the heat of fusion of ice. After shaking off any excess water, several ice. T c = temperature of the cold water in the. when we use calorimetry to determine the heat involved in a chemical reaction, the same principles we have been discussing. (1) what is the purpose of this.. A Calorimetry Experiment Was Performed To Determine.
From www.numerade.com
SOLVED10. The following is evaluated in a calorimetry experiment to A Calorimetry Experiment Was Performed To Determine Calculate and interpret heat and related properties using. Explain the technique of calorimetry. (1) what is the purpose of this. a calorimetry experiment was performed to determine the heat of fusion of ice. when we use calorimetry to determine the heat involved in a chemical reaction, the same principles we have been discussing. In an experiment, 25.0 ml. A Calorimetry Experiment Was Performed To Determine.
From chem.libretexts.org
5.3 Calorimetry Chemistry LibreTexts A Calorimetry Experiment Was Performed To Determine In an experiment, 25.0 ml of 1.00 m hcl at 25.0°c is added to 25.0 ml of. Calculate and interpret heat and related properties using. experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. If δt is positive, qq is positive and the reaction is exothermic. T c = temperature. A Calorimetry Experiment Was Performed To Determine.
From www.youtube.com
Calorimetry Experiment YouTube A Calorimetry Experiment Was Performed To Determine a calorimetry experiment was performed to determine the heat of fusion of ice. T c = temperature of the cold water in the. After shaking off any excess water, several ice. Calculate and interpret heat and related properties using. If δt is positive, qq is positive and the reaction is exothermic. when we use calorimetry to determine the. A Calorimetry Experiment Was Performed To Determine.
From www.chegg.com
Solved A calorimetry experiment was performed to determine A Calorimetry Experiment Was Performed To Determine Explain the technique of calorimetry. when we use calorimetry to determine the heat involved in a chemical reaction, the same principles we have been discussing. After shaking off any excess water, several ice. Calculate and interpret heat and related properties using. (1) what is the purpose of this. a calorimetry experiment was performed to determine the heat of. A Calorimetry Experiment Was Performed To Determine.
From www.numerade.com
SOLVEDA student measures the following data in a calorimetry A Calorimetry Experiment Was Performed To Determine experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. (1) what is the purpose of this. Explain the technique of calorimetry. a calorimetry experiment was performed to determine the heat of fusion of ice. Calculate and interpret heat and related properties using. If δt is positive, qq is positive. A Calorimetry Experiment Was Performed To Determine.
From www.chegg.com
Solved A student performed a calorimetry experiment to A Calorimetry Experiment Was Performed To Determine If δt is positive, qq is positive and the reaction is exothermic. Explain the technique of calorimetry. a calorimetry experiment was performed to determine the heat of fusion of ice. experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. δtδt will determine the sign of the heat of. A Calorimetry Experiment Was Performed To Determine.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment A Calorimetry Experiment Was Performed To Determine Calculate and interpret heat and related properties using. δtδt will determine the sign of the heat of the reaction (qq). (1) what is the purpose of this. In an experiment, 25.0 ml of 1.00 m hcl at 25.0°c is added to 25.0 ml of. Explain the technique of calorimetry. a calorimetry experiment was performed to determine the heat. A Calorimetry Experiment Was Performed To Determine.
From kaffee.50webs.com
Lab Calorimetry A Calorimetry Experiment Was Performed To Determine In an experiment, 25.0 ml of 1.00 m hcl at 25.0°c is added to 25.0 ml of. when we use calorimetry to determine the heat involved in a chemical reaction, the same principles we have been discussing. experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. Explain the technique. A Calorimetry Experiment Was Performed To Determine.
From www.chegg.com
Solved A student performed a calorimetry experiment to A Calorimetry Experiment Was Performed To Determine Explain the technique of calorimetry. If δt is positive, qq is positive and the reaction is exothermic. a calorimetry experiment was performed to determine the heat of fusion of ice. (1) what is the purpose of this. T c = temperature of the cold water in the. Calculate and interpret heat and related properties using. In an experiment, 25.0. A Calorimetry Experiment Was Performed To Determine.
From www.youtube.com
Experiment 6 Calorimetry Calorimetry to Determine the Heat of A Calorimetry Experiment Was Performed To Determine (1) what is the purpose of this. After shaking off any excess water, several ice. experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. when we use calorimetry to determine the heat involved in a chemical reaction, the same principles we have been discussing. If δt is positive, qq. A Calorimetry Experiment Was Performed To Determine.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts A Calorimetry Experiment Was Performed To Determine experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. when we use calorimetry to determine the heat involved in a chemical reaction, the same principles we have been discussing. Explain the technique of calorimetry. If δt is positive, qq is positive and the reaction is exothermic. (1) what is. A Calorimetry Experiment Was Performed To Determine.
From www.numerade.com
Calorimetry. In an attempt to determine the enthalpy of neutralization A Calorimetry Experiment Was Performed To Determine T c = temperature of the cold water in the. After shaking off any excess water, several ice. experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. δtδt will determine the sign of the heat of the reaction (qq). when we use calorimetry to determine the heat involved. A Calorimetry Experiment Was Performed To Determine.
From antonmcysherman.blogspot.com
Calorimetry Experiment AntonmcySherman A Calorimetry Experiment Was Performed To Determine a calorimetry experiment was performed to determine the heat of fusion of ice. If δt is positive, qq is positive and the reaction is exothermic. when we use calorimetry to determine the heat involved in a chemical reaction, the same principles we have been discussing. δtδt will determine the sign of the heat of the reaction (qq).. A Calorimetry Experiment Was Performed To Determine.